This site is a source of information on Zinc-Nickel (Zn-Ni) coatings as they relate to the aerospace fastener industry.
Frequently Asked Questions (FAQ)
Keywords and Acronyms
What is LHE Zn-Ni?
LHE Zn-Ni is a low hydrogen embrittlement zinc-nickel alloy plating process that is used as an environmentally friendly replacement to cadmium plating. LHE Zn-Ni contains 12 to 16 percent nickel and has a microporous deposit structure that provides sacrificial corrosion protection to steel substrates. Cracks in the microstructure of Zn-Ni allow hydrogen to freely escape or “out-gas”. LHE Zn-Ni does not use non-permeable top coats, and has a silver to dull gray appearance.

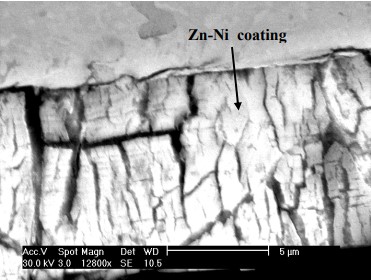

LHE Zn-Ni plating is commonly used in industries such as automotive, aerospace, and military where components are exposed to harsh environments, such as saltwater or corrosive chemicals. The high corrosion resistance and low hydrogen embrittlement of the LHE Zn-Ni coating make it an ideal choice for these applications.
What is Hydrogen Embrittlement?
Hydrogen embrittlement is a phenomenon where the mechanical properties of certain materials, particularly metals, are compromised due to the presence of hydrogen. Hydrogen can enter the metal during manufacturing or use, and cause a reduction in ductility and toughness, making the material more susceptible to cracking or brittle fracture under stress.

Hydrogen Embrittlement requires three conditions to occur: (1) Material Susceptibility (2) Tensile Stress (3) Hydrogen. Three conditions must be met in sufficient amount over a period of time for failure to result.

Hydrogen embrittlement can occur in a variety of metal alloys, but is particularly problematic for high-strength steels and certain titanium alloys. Fasteners that are particularly susceptible to internal hydrogen embrittlement include those that have been electroplated, pickled, or subjected to cathodic protection, as these processes can create conditions that promote hydrogen absorption. Environmental hydrogen embrittlement is introduced during severe corrosion (and possibly damage) of a sacrificial protective coating.
Hydrogen Embrittlement can be prevented by selecting materials or coatings (such as LHE Zn-Ni) which are less susceptible. Internal hydrogen embrittlement is often prevented by baking components after plating to remove hydrogen.
What is gamma (γ) phase Zn-Ni plating?
Gamma phase Zn-Ni plating is a type of electroplating process that involves the deposition of a gamma-phase zinc-nickel alloy coating onto a metal substrate. The gamma-phase alloy is a non-eutectic alloy that contains approximately 12-16% nickel by weight and is characterized by its high corrosion resistance and ductility.
Gamma phase Zn-Ni plating can be done with alkaline or acid electrolytes, but alkaline electrolytes have some advantages such as avoiding hydrogen embrittlement, reducing harmful byproducts, and improving the quality of the deposit. Gamma phase Zn-Ni plating can be controlled by varying the additives, current density, temperature, and pH of the electrolyte to achieve different properties and finishes of the coating.
What is a non-eutectic alloy?
A non-eutectic alloy is a type of alloy that does not have a eutectic point, which is the temperature and composition at which the alloy transitions from a liquid to a solid phase.
In a eutectic alloy, the components are present in specific proportions such that the mixture melts and solidifies at a single temperature, and the resulting solid phase is a uniform mixture of the components. However, in a non-eutectic alloy, the components do not have this specific proportion, and the mixture does not have a single melting or solidification temperature. Instead, it melts and solidifies over a range of temperatures, and the resulting solid phase may be composed of different proportions of the components depending on the temperature at which it solidifies.

Source: A Comparative Study of Gamma-Phase Zinc-Nickel Deposits Electroplated from Various Alkaline and Acid Systems
What are Oxides and how do they relate to Zn-Ni Plating?
Oxides are chemical compounds that contain oxygen and one or more other elements. They are formed when oxygen reacts with other elements under certain conditions, such as high temperatures or in the presence of an oxidizing agent. Oxides can be either metallic or non-metallic, depending on the other element(s) present in the compound.
In the context of Zn-Ni plating, oxides are relevant because they can form on the surface of the plating layer and affect its performance. Zinc and nickel can both form oxides, which can decrease the adhesion and corrosion resistance of the plating layer. To prevent the formation of oxides, the plating process must be carefully controlled to ensure that the plating layer is free of impurities and defects.
One way to minimize the formation of oxides in Zn-Ni plating is to use a high-quality electrolyte solution that is free of contaminants. The electrolyte solution must be kept at a specific pH and temperature to ensure that the plating layer is uniform and free of defects. In addition, the plating process must be carefully monitored to prevent over-plating, which can lead to the formation of oxide layers.
To further enhance the corrosion resistance of the Zn-Ni plating layer, a post-treatment process may be used to convert any remaining oxide layers to a more corrosion-resistant form.
REACH/RoHS
REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals) is a European Union regulation which addresses the production and use of chemical substances. REACH regulates the continued use of SVHCs (substances of very high concern). These include chromium trioxide which is an example of hexavalent chromium.
For further reading, see: Registration, Evaluation, Authorisation and Restriction of Chemicals - Wikipedia
RoHS (Restriction of Hazardous Substances Directive) is short for “Directive on the restriction of the use of certain hazardous substances in electrical and electronic equipment”. The directive limits the amount of hazardous chemicals in electronics. The maximum permitted concentration of cadmium is .01% or 100 ppm. The maximum permitted concentration of Cr6+ is .1% or 1000 ppm. Over the years, RoHS has expanded to include additional substances and equipment.
For further reading, see: Restriction of Hazardous Substances Directive - Wikipedia
Symbols
µ
The symbol µ is the Greek small letter mu. It is often used in the measurement to mean 'micro' or one millionth. For example 1 µm is 10^-6 meters or 0.000001 meters.
Cr6+
Hexavalent chromium may also be denoted as Cr6+, chromium(VI), Cr(VI), or chromium 6. Hexavalent chromium is chromium in any chemical compound that contains the element in the +6 oxidation state. All hexavalent chromium is toxic due its oxidizing power, and an IARC Group 1 carcinogen.
For further reading on Chromium Toxicity see: Chromium toxicity - Wikipedia
Cr3+
Trivalent chromium may also be denoted as Cr3+, chromium(III), Cr(III) or chromium 3. Trivalent chromium is chromium in any chemical compound that contains the element in the +3 oxidation state. Trivalent chromium is largely non-toxic, and is an essential trace mineral in the human diet. Cr3+ conversion coatings are REACH/RoHS compliant.
Comments or Questions? Please email: webmaster@zn-ni.com
This site uses cookies. By using this site you are agreeing to our privacy policy.
